Minetosh online
Geology – Web-Apps – Learning German – and more ...
Minetosh online – Geology Web-Apps
AmphiCalc-1: Amp13RM (Ca hornblendes)
Amp13RM – Amphibole compositions based on 13 cations without Na, K, Mn and Ca
Amp13RM allows the calculation of amphibole compositions based on 13 cations without sodium, potassium, manganese and calcium.
This 13 cation (- Na, K, Mn, Ca) method for amphibole calculation was proposed for the first time by Holland & Richardson (1979). The distribution of Ca and Na to the A and B (M4) positions is particularly suitable for Ca hornblendes. Here, too, the positions are filled according to LEAKE (1978).
By entering values into the \"% Fe₂O₃\" input-field, an iterative Fe³⁺ adjustment must be carried out until the sum of the valences equals 46.
Using microprobe analyses, Amp13RM can be used to automatically calculate amphibole compositions, the oxides, the cations, the lattice site occupancies, the values for divalent and trivalent iron (total iron, Fe²⁺, Fe³⁺) and more. The following diagrams are generated: Leake-diagram, ACF-diagram, AFM-diagram.
You can enter the values individually directly into the
table
below or use the
input fields
. Here you enter for up to 5 amphiboles the values (oxides) as rows separated by single spaces (see placeholders; 200 characters max.). Note the order of the elements in the table: Na Mg Al Si K Ca Ti Mn Fe (total Fe as FeO) H₂O! Additionally, the sample designation must come first. You can use the period or the comma as a decimal separator.
Literature see bottom of page
.
Technical note: Although the responsive design should work on all common screen sizes, this page is displayed best from a screen/window size of at least 1361px.
Amp13RM
Your amphiboles
You can also enter your data (oxides) here (into the light-blue fields). Here, you may also enter values for Fe₂O₃ manually.
| Name | |||||
| Oxides | Oxides | Oxides | Oxides | Oxides | |
| Na2O | |||||
| MgO | |||||
| Al2O3 | |||||
| Fe2O3 | |||||
| SiO2 | |||||
| K2O | |||||
| CaO | |||||
| TiO2 | |||||
| MnO | |||||
| FeO | |||||
| H2O | |||||
| V2O3 | |||||
| Total | |||||
| FeO total |
Calculation
Step 1: Fe³⁺-Determination
By manually entering values for the Fe₂O₃ content of total iron into the "% Fe₂O₃" fields, an iterative Fe³⁺ adjustment must be performed until the sum of the valences is 46 (the difference between the target and actual values must be 0). When entering values, please be sure to confirm the entries in the "% Fe₂O₃" field with the Enter or Tab key.
Notice: For the example data for amphiboles 1 to 4, values for the Fe₂O₃ content are already predefined (22.304%, 16.9%, 61.445%, and 25.019%). After clicking the "Calculation" button, the value for each valence should be 46, and the confirmation message "OK!" should appear. If this is not the case, please reload the page.
|
Enter Fe₂O₃ proportion of the total iron below |
Sum of valences(Required value: 46) |
Difference between the target and actual values (Target: 0) |
|||
| Name | % Fe2O3 | Valences | Valences | Difference | Check |
Step 2: Calculation
Click the button to determine the Fe₂O₃ content of the total iron and to calculate the amphibole composition.
Constants and intermediate results
| Element | Mol wt. (AW) | Mol-Prop. | Wt.-% | Atom-Prop. | Oxygen |
|---|---|---|---|---|---|
| Amphibole 1 | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe2+ | |||||
| Na | |||||
| K | |||||
| Fe3+ | |||||
| Fkt. O=23 | Total | ||||
| FM | |||||
| Amphibole 2 | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe2+ | |||||
| Na | |||||
| K | |||||
| Fe3+ | |||||
| Fkt. O=23 | Total | ||||
| FM | |||||
| Amphibole 3 | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe2+ | |||||
| Na | |||||
| K | |||||
| Fe3+ | |||||
| Fkt. O=23 | Total | ||||
| FM | |||||
| Amphibole 4 | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe2+ | |||||
| Na | |||||
| K | |||||
| Fe3+ | |||||
| Fkt. O=23 | Total | ||||
| FM | |||||
| Amphibol 5 | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe2+ | |||||
| Na | |||||
| K | |||||
| Fe3+ | |||||
| Fkt. O=23 | Total | ||||
| FM | |||||
| IM1 – Oxides | IM2 – Element |
|---|---|
| Amphibole 1 | |
| SiO2 | Si |
| TiO2 | Ti |
| Al2O3 | Al |
| V2O3 | Mg |
| MgO | Ca |
| CaO | Mn |
| MnO | Fe2+ |
| FeO | Na |
| Na2O | K |
| K2O | Fe3+ |
| Fe2O3 | V |
| Total | Total |
| Amphibole 2 | |
| SiO2 | Si |
| TiO2 | Ti |
| Al2O3 | Al |
| V2O3 | Mg |
| MgO | Ca |
| CaO | Mn |
| MnO | Fe2+ |
| FeO | Na |
| Na2O | K |
| K2O | Fe3+ |
| Fe2O3 | V |
| Total | Total |
| Amphibole 3 | |
| SiO2 | Si |
| TiO2 | Ti |
| Al2O3 | Al |
| V2O3 | Mg |
| MgO | Ca |
| CaO | Mn |
| MnO | Fe2+ |
| FeO | Na |
| Na2O | K |
| K2O | Fe3+ |
| Fe2O3 | V |
| Total | Total |
| Amphibole 4 | |
| SiO2 | Si |
| TiO2 | Ti |
| Al2O3 | Al |
| V2O3 | Mg |
| MgO | Ca |
| CaO | Mn |
| MnO | Fe2+ |
| FeO | Na |
| Na2O | K |
| K2O | Fe3+ |
| Fe2O3 | V |
| Total | Total |
| Amphibole 1 | |
| SiO2 | Si |
| TiO2 | Ti |
| Al2O3 | Al |
| V2O3 | Mg |
| MgO | Ca |
| CaO | Mn |
| MnO | Fe2+ |
| FeO | Na |
| Na2O | K |
| K2O | Fe3+ |
| Fe2O3 | V |
| Total | Total |
| Amphibol 1 | Amphibol 2 | Amphibol 3 | Amphibol 4 | Amphibol 5 | |
|---|---|---|---|---|---|
| Cation calculation (I) | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| Fe³⁺ | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe²⁺ | |||||
| Na | |||||
| K | |||||
| Total | |||||
| Amphibol 1 | Amphibol 2 | Amphibol 3 | Amphibol 4 | Amphibol 5 | |
|---|---|---|---|---|---|
| Cation calculation (II) – "Re-Calculation" | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| Fe³⁺ | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe²⁺ | |||||
| Na | |||||
| K | |||||
| Total | |||||
| Valences | |||||
| Total cations (I) | |||||
| Total cations (II) (- Na, K, Ca, Mn) |
|||||
Grid occupation
| Amphibole 1> | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al oct. |
Si on T |
Al oct. |
Si+Al+Cr (T) |
Si+Al+V (T) |
||||||
| V Rest |
Si+Al on T |
V Rest |
Fe³⁺ -"- (T) |
Fe³⁺ -"- (T) |
||||||
| Fe³⁺ Rest |
Space on T |
Fe³⁺ Rest |
V on T |
V on T |
||||||
| Fe³⁺ on T |
Al on T |
Al on T |
||||||||
| Mg Rest |
M1+M2 |
Mg Rest |
Mg on C |
|||||||
| Fe²⁺ Rest |
M1+M2+Mg |
Fe²⁺ Rest |
Fe²⁺ on C |
|||||||
| Mn²⁺ Rest |
M1+M2+Mg+Fe²⁺ |
Mn²⁺ Rest |
Mn²⁺ on C |
|||||||
| M4 | ||||||||||
| Na Rest on A |
Mg+Fe+Mn+Ca |
Na Rest on A |
||||||||
| Space on B |
Na on B |
|||||||||
| ACF (Mol Prop.) | ||||||||||
| A | ||||||||||
| C | ||||||||||
| F | ||||||||||
| Σ | ||||||||||
| AFM (Ms-Project) | ||||||||||
| A | ||||||||||
| F | ||||||||||
| M | ||||||||||
| Formula occupation | ||||||||||
| T | ||||||||||
| C (M1-M3) | ||||||||||
| B (M4) | ||||||||||
| A | ||||||||||
| Amphibole 2> | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al oct. |
Si on T |
Al oct. |
Si+Al+Cr (T) |
Si+Al+V (T) |
||||||
| V Rest |
Si+Al on T |
V Rest |
Fe³⁺ -"- (T) |
Fe³⁺ -"- (T) |
||||||
| Fe³⁺ Rest |
Space on T |
Fe³⁺ Rest |
V on T |
V on T |
||||||
| Fe³⁺ on T |
Al on T |
Al on T |
||||||||
| Mg Rest |
M1+M2 |
Mg Rest |
Mg on C | |||||||
| Fe²⁺ Rest |
M1+M2+Mg |
Fe²⁺ Rest |
Fe²⁺ on C |
|||||||
| Mn²⁺ Rest |
M1+M2+Mg+Fe²⁺ |
Mn²⁺ Rest |
Mn²⁺ on C |
|||||||
| M4 | ||||||||||
| Na Rest on A |
Mg+Fe+Mn+Ca |
Na Rest on A |
||||||||
| Space on B |
Na on B |
|||||||||
| ACF (Mol Prop.) | ||||||||||
| A | ||||||||||
| C | ||||||||||
| F | ||||||||||
| Σ | ||||||||||
| AFM (Ms-Project) | ||||||||||
| A | ||||||||||
| F | ||||||||||
| M | ||||||||||
| Formula occupation | ||||||||||
| T | ||||||||||
| C (M1-M3) | ||||||||||
| B (M4) | ||||||||||
| A | ||||||||||
| Amphibole 3> | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al oct. |
Si on T |
Al oct. |
Si+Al+Cr (T) |
Si+Al+V (T) |
||||||
| V Rest |
Si+Al on T |
V Rest |
Fe³⁺ -"- (T) |
Fe³⁺ -"- (T) |
||||||
| Fe³⁺ Rest |
Space on T |
Fe³⁺ Rest |
V on T |
V on T |
||||||
| Fe³⁺ on T |
Al on T |
Al on T |
||||||||
| Mg Rest |
M1+M2 |
Mg Rest |
Mg on C |
|||||||
| Fe²⁺ Rest |
M1+M2+Mg |
Fe²⁺ Rest |
Fe²⁺ on C |
|||||||
| Mn²⁺ Rest |
M1+M2+Mg+Fe²⁺ |
Mn²⁺ Rest |
Mn²⁺ on C |
|||||||
| M4 | ||||||||||
| Na Rest on A |
Mg+Fe+Mn+Ca |
Na Rest on A |
||||||||
| Space on B |
Na on B |
|||||||||
| ACF (Mol Prop.) | ||||||||||
| A | ||||||||||
| C | ||||||||||
| F | ||||||||||
| Σ | ||||||||||
| AFM (Ms-Project) | ||||||||||
| A | ||||||||||
| F | ||||||||||
| M | ||||||||||
| Formula occupation | ||||||||||
| T | ||||||||||
| C (M1-M3) | ||||||||||
| B (M4) | ||||||||||
| A | ||||||||||
| Amphibole 4> | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al oct. |
Si on T |
Al oct. |
Si+Al+Cr (T) |
Si+Al+V (T) |
||||||
| V Rest |
Si+Al on T |
V Rest |
Fe³⁺ -"- (T) |
Fe³⁺ -"- (T) |
||||||
| Fe³⁺ Rest |
Space on T |
Fe³⁺ Rest |
V on T |
V on T |
||||||
| Fe³⁺ on T |
Al on T |
Al on T |
||||||||
| Mg Rest |
M1+M2 |
Mg Rest |
Mg on C |
|||||||
| Fe²⁺ Rest |
M1+M2+Mg |
Fe²⁺ Rest |
Fe²⁺ on C |
|||||||
| Mn²⁺ Rest |
M1+M2+Mg+Fe²⁺ |
Mn²⁺ Rest |
Mn²⁺ on C |
|||||||
| M4 | ||||||||||
| Na Rest on A |
Mg+Fe+Mn+Ca |
Na Rest on A |
||||||||
| Space on B |
Na on B |
|||||||||
| ACF (Mol Prop.) | ||||||||||
| A | ||||||||||
| C | ||||||||||
| F | ||||||||||
| Σ | ||||||||||
| AFM (Ms-Project) | ||||||||||
| A | ||||||||||
| F | ||||||||||
| M | ||||||||||
| Formula occupation | ||||||||||
| T | ||||||||||
| C (M1-M3) | ||||||||||
| B (M4) | ||||||||||
| A | ||||||||||
| Amphibole 5> | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Al oct. |
Si on T |
Al oct. |
Si+Al+Cr (T) |
Si+Al+V (T) |
||||||
| V Rest | Si+Al on T |
V Rest |
Fe³⁺ -"- (T) |
Fe³⁺ -"- (T) |
||||||
| Fe³⁺ Rest |
Space on T |
Fe³⁺ Rest |
V on T |
V on T |
||||||
| Fe³⁺ on T |
Al on T |
Al on T |
||||||||
| Mg Rest |
M1+M2 |
Mg Rest |
Mg on C |
|||||||
| Fe²⁺ Rest |
M1+M2+Mg |
Fe²⁺ Rest |
Fe²⁺ on C |
|||||||
| Mn²⁺ Rest |
M1+M2+Mg+Fe²⁺ |
Mn²⁺ Rest |
Mn²⁺ on C |
|||||||
| M4 | ||||||||||
| Na Rest on A |
Mg+Fe+Mn+Ca |
Na Rest on A |
||||||||
| Space on B |
Na on B |
|||||||||
| ACF (Mol Prop.) | ||||||||||
| A | ||||||||||
| C | ||||||||||
| F | ||||||||||
| Σ | ||||||||||
| AFM (Ms-Project) | ||||||||||
| A | ||||||||||
| F | ||||||||||
| M | ||||||||||
| Formula occupation | ||||||||||
| T | ||||||||||
| C (M1-M3) | ||||||||||
| B (M4) | ||||||||||
| A | ||||||||||
| Amphibol 1 | Amphibol 2 | Amphibol 3 | Amphibol 4 | Amphibol 5 | |
|---|---|---|---|---|---|
| Intermediate cation occupation | |||||
| Si | |||||
| Al tetr. | |||||
| V on T | |||||
| Fe³⁺ tetr. | |||||
| Total T | |||||
| V Rest | |||||
| Al oct. | |||||
| Ca | |||||
| Fe³⁺ Rest | |||||
| Ti | |||||
| Mg (M3) | |||||
| Fe²⁺ (M3) | |||||
| Mn²⁺ (M3) | |||||
| Total C | |||||
| Mg Rest | |||||
| Fe²⁺ Rest | |||||
| Mn²⁺ Rest | |||||
| Ca | |||||
| Na on B | |||||
| Total B | |||||
| Na Rest on A | |||||
| K | |||||
| Total A | |||||
| Na on B | |||||
| Mg/Mg²⁺+Fe²⁺ | |||||
| Fe³⁺/Fe³⁺+Al⁶⁺ | |||||
| Si on T | |||||
| Amphibol 1 | Amphibol 2 | Amphibol 3 | Amphibol 4 | Amphibol 5 | |
|---|---|---|---|---|---|
| Even more intermediate results (for control purposes) | |||||
| F1 | |||||
| F100 | |||||
| SiO₂ | |||||
| TiO₂ | |||||
| Al₂O₃ | |||||
| V₂O₃ | |||||
| MgO | |||||
| CaO | |||||
| MnO | |||||
| FeO | |||||
| Na₂O | |||||
| K₂O | |||||
| Fe₂O₃ | |||||
| Total | |||||
| Al tetr. | |||||
| V tetr. | |||||
| Fe³⁺ tetr. | |||||
| M1+M2 | |||||
| Mg (M3) | |||||
| Fe²⁺ (M3) | |||||
| Mn²⁺ (M3) | |||||
| Na (M4) | |||||
| Na on B (M4) | |||||
| Na on A | |||||
| Mg, Fe, Mn, Na | |||||
| Mg/Mg+Fe²⁺ | |||||
| Fe³⁺/Fe³⁺+Al⁶⁺ | |||||
| Mg+Fe+Mn+Ca (M4) | |||||
| Fe₂O₃ init. | |||||
| Fe₂O₃ new | |||||
| Total (1) T | |||||
| Total (2) T | |||||
| Total (1) C | |||||
| Total (2) C | |||||
| Total (1) B | |||||
| Total (2) B | |||||
| Tetrahedron | |||||
| C (M1-M3) | |||||
| B (M4) | |||||
| A | |||||
| X Mg | |||||
| X Fe³⁺ | |||||
| ln (Ca/Na) on B | |||||
| Total A | |||||
| Fe³⁺/Al | |||||
| (Ca+Na)B | |||||
| Classifikation | |||||
| imma = Iron-magnesium-manganese amphibole; ca = Calcic amphibole; sca = Sodic-calcic amphibole; aa = Alkali amphibole | |||||
Results
Amphibole (23 oxygens)
| Amphibole 1 | Amphibole 2 | Amphibole 3 | Amphibole 4 | Amphibole 5 | |
|---|---|---|---|---|---|
| Fe₂O₃ re-calculation | |||||
| Fe₂O₃ Initial | |||||
| Fe₂O₃ New | |||||
| Classification after Leake | |||||
| Oxides | |||||
| SiO2 | |||||
| TiO2 | |||||
| Al2O3 | |||||
| Fe2O3 | |||||
| V2O3 | |||||
| MgO | |||||
| CaO | |||||
| MnO | |||||
| FeO | |||||
| Na2O | |||||
| K2O | |||||
| Total | |||||
| Cations (O=23) | |||||
| Si | |||||
| Ti | |||||
| Al | |||||
| Fe3+ | |||||
| V | |||||
| Mg | |||||
| Ca | |||||
| Mn | |||||
| Fe2+ | |||||
| Na | |||||
| K | |||||
| Total | |||||
| Formula occupation | |||||
| T | |||||
| Si | |||||
| Al | |||||
| V | |||||
| Fe3+ | |||||
| Total | |||||
| C (6) | |||||
| Al | |||||
| V | |||||
| Fe3+ | |||||
| Ti | |||||
| Mg | |||||
| Fe2+ | |||||
| Mn | |||||
| Total | |||||
| B | |||||
| Mg | |||||
| Fe2+ | |||||
| Mn | |||||
| Ca | |||||
| Na | |||||
| Total | |||||
| A | |||||
| Na | |||||
| K | |||||
| Total | |||||
| .... | |||||
| Na (B) | |||||
| Mg/(Mg+Fe2+) | |||||
| Fe3+/(Fe3++Al6+) | |||||
| Si/Cell | |||||
| ln(Ca/Na)B | |||||
| ACF | |||||
| A | |||||
| C | |||||
| F | |||||
| Total | |||||
| AFM | |||||
| A | |||||
| F | |||||
| M | |||||
| Fe₂O₃ proportion of the total iron | |||||
| % Fe2O3 | |||||
| Comments | |||||
The diagrams
The ACF diagram
The amphiboles in the ACF diagram. To download the chart (Amphibole_ACF.svg) and see more options, hover your mouse over the image. You can zoom in on any area. To zoom back to the original size, double-click the image.
Captions
|
• |
Amphibole 1 |
|
• |
Amphibole 2 |
|
• |
Amphibole 3 |
|
• |
Amphibole 4 |
|
• |
Amphibole 5 |
The AFM diagram
The amphiboles shown in the AFM(Ms) diagram (Top: A; Right: M; Left: F). After Nelson, S.A. (2011). To download the chart (Amphibole_AFM(Ms).svg) and see more options, hover your mouse over the image.
Captions
|
• |
Amphibole 1 |
|
• |
Amphibole 2 |
|
• |
Amphibole 3 |
|
• |
Amphibole 4 |
|
• |
Amphibole 5 |
|
Between the red lines |
Biotite |
|
Between the green lines |
Chlorite |
|
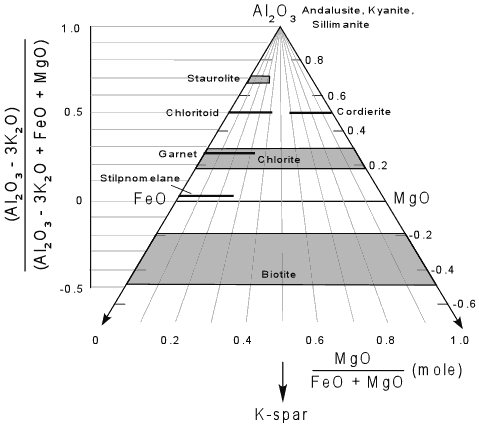
The AFM(Ms) diagram The AFM(Ms) diagram. From Nelson, S.A. (2011). |
|
The Leake diagram
The amphiboles shown in the Leake diagram (after Leake, B.E., 1978). To download the chart (Amphibole_Leake.svg) and see more options, hover your mouse over the image.
Captions
|
• |
Amphibole 1 |
|
• |
Amphibole 2 |
|
• |
Amphibole 3 |
|
• |
Amphibole 4 |
|
• |
Amphibole 5 |
|
The fields in the Leake diagram The fields in the Leake-Diagram (see below). |
|
| 1 | Tremolite |
| 2 | Tremolitic Hornblende |
| 3 | Magnesio-Hornblende |
| 4 | Tschermakitic Hornblende |
| 5 | Tschermakite (Alumino-Tschermakite) |
| 6 | Actinolite |
| 7 | Actinolitic Hornblende |
| 8 | Ferro-Actinolite |
| 9 | Ferro-Actinolitic Hornblende |
| 10 | Ferro-Hornblende |
| 11 | Ferro-Tschermakitic Hornblende |
| 12 | Ferro-Tschermakite |
Download data
Current date
Enter a title
Sample number, etc.
Download data file (*.csv)
Literature
- Deer, W.A., Howie, R.A. & Zussman, J. (1992): An introduction to the rock-forming minerals. – Longman Scientific & Technical, Harlow, 2nd edition 1992: 696 p.
- Ehlers, E.G. and Blatt, H. (1982): Petrology: Igneous, Sedimentary and Metamorphic. W. H. Freeman, New York, 732 p.
- Holland T.J.B. & Richardson S.W. (1979): Amphibole zonation in metabasites as a guide to the evolution of metamorphic conditions. - Contrib. Mineral. Petrol., 70: 143-148.
- Leake B.E. (1978): Nomenclature of amphiboles. - Am. Mineral., 63: 1023-1053.
- Matthes S. (1987): Mineralogie – Eine Einführung in die spezielle Mineralogie, Petrologie und Lagerstättenkunde. – Springer-Verlag, Berlin, Heidelberg, 1987: 444 p.
- Nelson, S.A. (2011): Triangular Plots in Metamorphic Petrology – EENS 2120; Tulane University: PDF EENS 2120.
- Robinson P., Spear F.S., Schumacher J.C., Laird J., Klein C., Evans B.W. & Doolan B.L. (1982): Phase relations of metamorphic amphiboles: natural ocurrenceand theory. In: Veblen D.R. & Ribbe P.H. (Eds.): Amphiboles: Petrology and experimental phase relations. - Reviews in mineralogy, 9b, Min. Soc. of America.
- Schäfer, J. (1996): Mikrosondenuntersuchungen an Geröllen und detritischen Mineralen im Flysch des Saxothuringikums: Ein Beitrag zur Exhumierungsgeschichte des Liefergebietes. – Inaugural dissertation to obtain a doctorate in natural sciences from the Justus Liebig University in Giessen (Department of Geosciences and Geography), Giessen, March 1996; 176 p.; German with English and French summaries.